Abstract
Acute myeloid leukemia (AML) is an aggressive cancer with a poor prognosis, for which the therapeutic landscape has changed little for decades. Aberrant mRNA splicing plays an important role in cancer development and genes coding for several of the major components of the spliceosome are targeted by somatic mutations in several cancers including myelodysplastic syndromes and AML. Recently, myeloid neoplasms harbouring spliceosome gene mutations were shown to be preferentially susceptible to pharmacological disruption of the spliceosome. Here we report that targeting particular pathways of the spliceosome machinery can also be an effective therapeutic strategy in other types of AML.
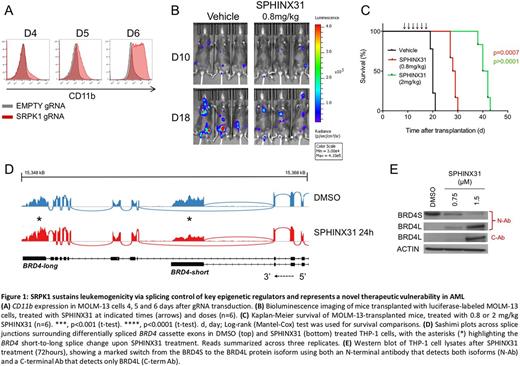
Recently, we generated a comprehensive catalogue of genetic vulnerabilities in AML using CRISPR-Cas9 genome-wide recessive screens and reported several novel intuitive and non-intuitive therapeutic candidates. Amongst these we identified SRPK1, the gene coding for a serine-threonine kinase that phosphorylates the major spliceosome protein SRSF1. Here, we demonstrate that targeted genetic disruption of SRPK1 in MLL-rearranged AMLs leads to differentiation and apoptosis (Fig. 1A). Additionally, the survival of immunocompromised mice transplanted with human AML cell lines carrying the MLL-AF9 fusion gene, namely MOLM-13 and THP-1, was significantly prolonged by genetic disruption of SRPK1 with CRISPR-Cas9. Similar effects were seen with pharmacological inhibition of SRPK1 in vitro and in vivo, using the novel SRPK1-specific kinase inhibitor SPHINX31 (Fig. 1B-C). Importantly, we go on to demonstrate that, while the SRPK1 kinase activity is required for AML cell survival, it is dispensable for normal hematopoiesis.
At the molecular level, we show that genetic or pharmacological inhibition of SRPK1 was associated with widespread changes in the splicing of multiple genes including several with roles in leukemogenesis such as MYB, BRD4 and MED24 . We focused on BRD4 as its splicing isoforms have distinct molecular properties and found that SRPK1 inhibition led to a substantial switch from the short (BRD4S) to the long (BRD4L) isoform at the mRNA and protein levels (Fig. 1D-E). This was associated with BRD4 eviction from genomic loci involved in myeloid leukemogenesis including BCL2 and MYC. Notably, ectopic expression of the short (BRD4S) isoform rescued the phenotype of SRPK1 inhibition suggesting that the observed BRD4 splicing switch mediates at least part of the anti-leukemic effects of SRPK1 inhibition. Furthermore, we show that the BRD inhibitor iBET-151 synergizes with SRPK1 inhibition to kill human MLL-AF9 -driven AMLs in vitro and in vivo.
Collectively our findings reveal that SRPK1 is required for normal splicing of key epigenetic regulators including BRD4 and represents a novel therapeutic vulnerability in AML that can be used alone or in combination with clinically relevant epigenetic drugs to enhance their anti-leukemic effects.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal